The Ozempic Lawsuit of 2025: Understanding the Risks
Introduction
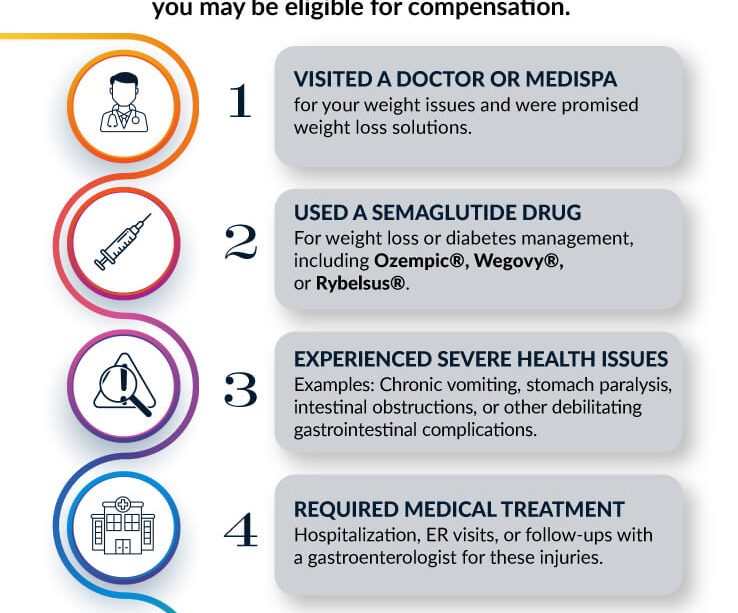
The Ozempic lawsuit of 2025 has emerged as a significant topic in the legal and health sectors, drawing attention from both patients and legal experts. Ozempic, a medication prescribed for Type 2 diabetes, has gained popularity for its effectiveness in weight loss. However, recent reports of severe side effects have prompted several lawsuits against the manufacturer, raising concerns regarding consumer safety and corporate responsibility.
Background on Ozempic
Approved by Health Canada in 2018, Ozempic (semaglutide) is recognized for helping manage blood sugar levels and promoting weight loss among individuals with obesity and diabetes. Despite its benefits, numerous patients have reported experiencing adverse effects, including severe gastrointestinal issues and potential links to thyroid cancer.
Details of the Lawsuit
As of early 2025, multiple lawsuits have been filed across Canada and the United States, alleging that the manufacturers, Novo Nordisk, failed to adequately warn consumers of the potential risks associated with long-term use of the drug. Plaintiffs are claiming negligence and seeking compensation for alleged pain and suffering, economic losses, and medical expenses incurred due to Ozempic-related health issues.
Legal experts predict that the lawsuits may focus on the thoroughness of clinical trials conducted during the drug’s approval process and the adequacy of existing warnings on packaging. Critics argue that the company prioritized market gains over patient safety, which may lead to significant legal repercussions.
Implications for Patients
For patients currently using Ozempic or considering its use, the ongoing lawsuits highlight the importance of staying informed and consulting healthcare professionals regarding any medication. Patients should be aware of the reported side effects and discuss alternatives if they express concerns about their treatment.
Conclusion
The developments surrounding the Ozempic lawsuit in 2025 underscore the growing scrutiny on pharmaceutical companies and the health implications of their products. As court cases unfold, patients and healthcare providers remain watchful, advocating for stronger regulatory measures to ensure drug safety and transparency. The outcome of these lawsuits may not only affect those directly involved but could also lead to a reevaluation of how medications are marketed and monitored in Canada. For consumers, the priority lies in being informed and maintaining open communication with their health professionals.







